Thermodynamics of Chemical Equilibrium:
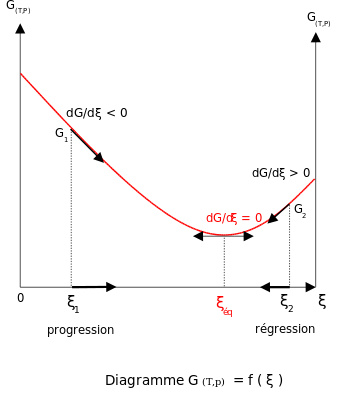
The relation between the Gibbs energy and the equilibrium constant can be found by considering chemical potentials.At constant temperature and pressure, the Gibbs free energy, G, for the reaction depends only on the extent of reaction: ξ (Greek letter xi), and can only decrease according to the second law of thermodynamics. It means that the derivative of G with ξ must be negative if the reaction happens; at the equilibrium the derivative being equal to zero.
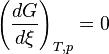 : equilibrium
: equilibrium
In this article only the constant pressure case is considered. The constant volume case is important in geochemistry and atmospheric chemistry where pressure variations are significant. Note that, if reactants and products were in standard state (completely pure), then there would be no reversibility and no equilibrium. The mixing of the products and reactants contributes a large entropy (known as entropy of mixing) to states containing equal mixture of products and reactants. The combination of the standard Gibbs energy change and the Gibbs energy of mixing determines the equilibrium state.
In general an equilibrium system is defined by writing an equilibrium equation for the reaction
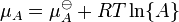 , (
, (  is the standard chemical potential ).
is the standard chemical potential ).
 in the case of a closed system.
in the case of a closed system.
 (
( 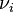 corresponds to the Stoichiometric coefficient and
corresponds to the Stoichiometric coefficient and 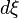 is the differential of the extent of reaction ).
is the differential of the extent of reaction ).
 which corresponds to the Gibbs free energy change for the reaction .
which corresponds to the Gibbs free energy change for the reaction .
 .
.
 ,
,
-
 : which is the standard Gibbs energy change for the reaction. It is a constant at a given temperature, which can be calculated, using thermodynamical tables.
: which is the standard Gibbs energy change for the reaction. It is a constant at a given temperature, which can be calculated, using thermodynamical tables.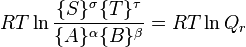
- (
 is the reaction quotient when the system is not at equilibrium ).
is the reaction quotient when the system is not at equilibrium ).
- (

-
 ; the reaction quotient becomes equal to the equilibrium constant.
; the reaction quotient becomes equal to the equilibrium constant.
Addition of reactants or products
For a reactional system at equilibrium: ;
; 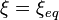 .
.- If are modified activities of constituents, the value of the
reaction quotient changes and becomes different from the equilibrium
constant:
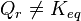

and

then
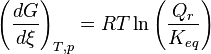
- If activity of a reagent
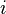 increases
increases
 , the reaction quotient decreases.
, the reaction quotient decreases.- then
 and
and 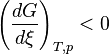 : The reaction will shift to the right (i.e. in the forward direction, and thus more products will form).
: The reaction will shift to the right (i.e. in the forward direction, and thus more products will form).- If activity of a product
 increases
increases
- then
 and
and  : The reaction will shift to the left (i.e. in the reverse direction, and thus less products will form).
: The reaction will shift to the left (i.e. in the reverse direction, and thus less products will form).Note that activities and equilibrium constants are dimensionless numbers.
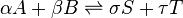
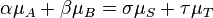
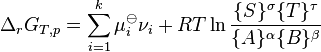



No comments:
Post a Comment